Research interest in the stable synthesis of multimetallic compounds—complex molecules containing multiple metallic atoms—has recently surged owing to their vast potential in catalysis, energy, and medicine. Employing the concept of orbital hybridization where the constituent atomic orbitals are mixed together into hybrids, bi- and tri-metallic nanoclusters have been synthesized and explored. Going beyond tri-metallic nanoclusters, however, has proven challenging thus far, particularly so for metals that show thermodynamically unfavorable alloy formation.
Syntheses have usually led to the unwanted outcome of phase separation of the various metal atoms. As an additional problem, the nanoclusters produced are often heterogeneous in size, where researchers want highly monodispersed nanoclusters for various applications.
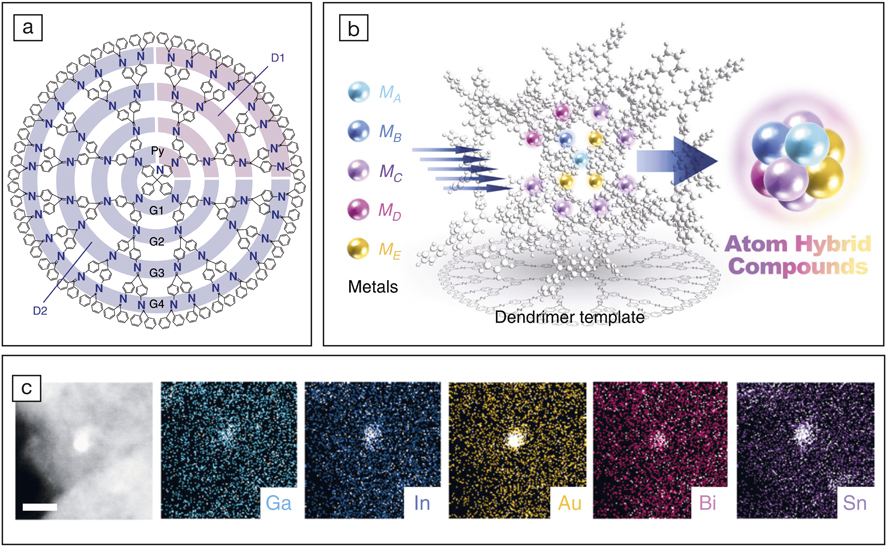
(a) Dendrimer employed as a template for metal accumulation; (b) schematic showing accumulation of various metals on the template; and (c) high-resolution scanning transmission electron microscopy/energy-dispersive x-ray spectrometry mapping of the Ga1In1Au3Bi2Sn6 cluster confirming absence of phase separation. Scale bar is 5 nm. Credit: Nature Communications.
This challenge has now been overcome by the research group of Kimihisa Yamamoto at the Tokyo Institute of Technology. As described in an article recently published in Nature Communications (doi:10.1038/s41467-018-06422-8), Yamamoto and co-workers employed dendrimers—a specific class of branched molecules—as templates to bring together atoms of as many as five different metals. The resulting multimetallic clusters are less than a nanometer in size. The researchers realized that blending the metals on a subnanometer scale (2–30 atoms) would thermodynamically stabilize chemical bond formation between the various metals, due to the presence of disordered phases and fewer nearest neighbors at these ultralow dimensions, circumventing phase separation.
“We have proposed a new concept called ‘atom hybridization.’ If a lot of metal elements are freely accumulated in the quantum-size regime, a totally new feature of the elements will emerge due to atomic orbital mixing,” Yamamoto says. “The hybridization, in this case,” he continues, “will exhibit its characteristic features mostly in the subnanometer realm with the atomicity of 2–30. This is the concept behind atom hybridization. It was enabled for the first time by using our new dendrimers [dendritic polyphenylazomethines] with potential gradient, which is different from the conventional dendrimer analogues.”
The dendrimer template used in this study exhibits a radially varying electric field that helps to selectively accumulate metal atoms depending on their electronegativity. Accumulation of the various metal atoms on the dendrimer was confirmed by observation of successive changes to the absorption spectrum. One such sub-nanocluster was Ga1In1Au3Bi2Sn6 containing a total of 13 atoms with a mean diameter of 0.94 nm. Importantly, a small standard deviation of 0.10 nm confirmed the low size-polydispersity. High-resolution scanning transmission electron microscope (STEM) images of the clusters with energy-dispersive x-ray spectrometry (EDS) mapping confirmed the absence of phase separation and indicated that the various metal atoms were uniformly distributed.
In addition to the demonstration of five-metal clusters, the researchers showed that as many as eight different metal atoms could be accumulated on the template according to the choice of dendrimer. The importance of the dendrimer-assisted synthesis was also further highlighted by a control experiment, where the synthesis was carried out without the dendrimer template. Wide polydispersity in the cluster size was found with significant phase separation.
Yamamoto says that these findings can potentially lead to a large range of polymetallic functional nanomaterials that have yet to be synthesized. “There are more than 90 metals in all 118 elements. Evidently this means the combinations of atom hybridization are almost infinite, and so are the multinary clusters considering the atomicity. We believe this concept and methodology will open up a new field in chemistry, materials science, and nanotechnology, leading to creation of as-yet-unknown materials,” Yamamoto says.




