Introduction
Mangrove fan palm (Licuala spinosa Thunb.) is a native to wet and salt water land of Southeast Asia that belongs to the family Arecaceae in the genus Licuala. It is a clump-forming ornamental palm and well-adapted to southern Thailand, especially in peat swamp forest. The plant has a great agronomic and socio-economic importance because the leaf can be used to make many products in handicraft such as hat, bag and fan, etc. Thai people use it during important religious festivals such as the end of the Buddhist Lent, the Chak Phra Festival, the Festival of the Tenth Lunar Month (Sart Duan Sip) and Hari Raya Day of Islam people. So far, the plant is decreasing due to industrial development, urbanization and changing the original area for growing economic crops. Normally, this plant is propagated by seed and sucker. On the other hand, this palm species has a slow growth and is difficult to propagate in mass production. Thus, tissue culture technique can help the production of a large number of plants within a short time. This will help this palm species to not become extinct from natural habitats and meanwhile this technique can be used to preserve germplasm in vitro. In vitro propagation of palm trees through somatic embryogenesis has been achieved (Gomes et al., Reference Gomes, Bartos and Scherwinski-Pereira2017; Mazri et al., Reference Mazri, Belkoura, Meziani, Mokhless and Nour2017; Heedchim et al., Reference Heedchim, Te-chato and Yenchon2020, Reference Heedchim, Te-chato and Yenchon2021; Stefenon et al., Reference Stefenon, Ree, Pinheiro, Goeten, Steiner and Guerra2020).
Zygotic embryo (ZE) is an excellent source of explant that has a great potential to develop into somatic embryo (SE) due to high responsiveness, abundance, high availability and high degree of physiological uniformity (Ree and Guerra, Reference Ree and Guerra2015). It has been used as a starting plant material for somatic embryogenesis in many plant species including palm trees such as Euterpe precatoria Mart (Ferreira et al., Reference Ferreira, Silva-Cardoso, Meira and Scherwinski-Pereira2022a) oil palm (Weckx et al., Reference Weckx, Inzé and Maene2019; Heedchim et al., Reference Heedchim, Te-chato and Yenchon2020; Niha et al., Reference Niha, Te-chato and Yenchon2022), Areca catechu L. (Wang et al., Reference Wang, Chen, Wu, Lin and Chang2003), Euterpe edulis (Saldanha and Martins-Corder, Reference Saldanha and Martins-Corder2012), Butia odorata (Campos et al., Reference Campos, Scherwinski-Pereira, Bernd, Fior and Schwarz2020) and Eleiodoxa conferta (Griff.) Burr. (Kanjanasopa et al., Reference Kanjanasopa, Somwong, Srisawat, Thitithanakul, Sontikul and Choengthong2017). Somatic embryogenesis of Arecaceae requires exogenous auxins such as 2,4-dichlorophenoxyacetic acid (2,4-D), dicamba or picloram to induce SE as reported by many researchers (Hassan and Taha, Reference Hassan and Taha2012; Intha et al., Reference Intha, Sujipuli, Chareonsap and Chaiprasart2016; Mazri et al., Reference Mazri, Belkoura, Meziani, Mokhless and Nour2017; Meira et al., Reference Meira, Luis, Silva-Cardoso and Scherwinski-Pereira2019; Hassan et al., Reference Hassan, Allam, Din, Malhat and Taha2021; Ferreira et al., Reference Ferreira, Silva-Cardoso, Meira and Scherwinski-Pereira2022a). Bonetti et al. (Reference Bonetti, Nesi, Quisen and Quoirin2016) reported that ZE cultured on 500 μM 2,4-D containing MS medium gave the highest callus induction in oil palm after 90 days of culture. The highest embryogenic callus induction at 93.3% for E. precatoria was obtained on 13.57 μM picloram containing medium after culturing ZE for 90 days (Ferreira et al., Reference Ferreira, Silva-Cardoso, Meira and Scherwinski-Pereira2022a). However, there is no report on in vitro propagation of mangrove fan palm because of difficulty of high phenolic compound production. Therefore, it is necessary to study factors affecting in vitro propagation of this palm. The aims of this research were to study effects of auxins and embryo cutting on plant regeneration of mangrove fan palm through indirect somatic embryogenesis.
Materials and methods
Plant material
Immature seed of mangrove fan palm (Fig. 1(a)) was collected from Thepa district, Songkhla province, Thailand, washed with detergent solution (Teepol) to clean the seed, again washed with running tap water for 30 min, soaked in 70% alcohol for 1 min followed by soaking in 20% Clorox with 150 μl/100 ml of Tween 20 for 20 min, then washed with sterile distilled water for three times, and placed on sterile tissue paper to dry the seed. The seed coat was removed using scalpel and endosperm together with immature zygotic embryo (IZE) was excised to culture on solidified oil palm culture medium (OPCM) with 200 mg/l ascorbic acid, but without plant growth regulators (PGRs).

Figure 1. Development of nodular callus from culturing IZE of mangrove fan palm (bar = 0.5 cm). (a) Immature seed used as starting plant material. (b) Germinated ZE on PGR-free MS for 7–10 days. (c) Nodular callus induced on MS with 1 mg/l dicamba. (d) Chopped nodular callus proliferated on 0.1 mg/l dicamba containing OPCM.
Culture media and conditions
The medium used in callus, secondary somatic embryo (SSE) induction and germination was full strength MS medium. For callus proliferation, OPCM which was modified by the combination of Murashige and Skoog (MS) and Woody Plant Medium (WPM), was used according to the method of Kerdsuwan and Te-chato (Reference Kerdsuwan and Te-chato2016). All culture media were added with 3% sucrose, adjusted to pH 5.7 and solidified with 0.6% agar before autoclaving at 1.05 kg/cm2, 121°C for 15 min. In case of ascorbic acid, it was sterilized by filtering through microfiltration with a 0.45 μm pore size under vacuum condition and added directly to the medium after autoclaving and cool down to 45–50°C.
Callus induction
Effects of different types and concentrations of auxins on callus induction
IZE was excised from endosperm after 7–10 days (Fig. 1(b)) of culture then directly transferred to MS medium supplemented with different concentrations of 2,4-D or dicamba with 200 mg/l ascorbic acid. The samples were placed in 25 × 150 mm test tubes containing 10 ml solidified medium and maintained under dark condition at 26 ± 2°C for 8 weeks. The callus induction frequency, callus diameter and characteristics of callus were investigated. Completely randomized design (CRD) with five replications was performed. Each replication consists of five test tubes and means among treatments were compared by Duncan's multiple range test (DMRT) at 1 or 5% probability.
Effect of embryo preparation on callus induction
The IZE at 7–10 days from culturing seed was prepared into two types; no wounding or cutting and longitudinally dissected into half. Both types of prepared IZEs were placed on 10 ml of solidified MS supplemented with 1 mg/l dicamba and 200 mg/l ascorbic acid contained in 25 × 150 mm test tubes. The cultures were maintained under dark condition at 26 ± 2°C for 8 weeks. The callus induction frequency, diameter and characteristics of callus were investigated. CRD with five replications was performed. Each replication consists of five test tubes and means among treatments were compared by t-test at 1 or 5% probability.
Effects of chopping and concentrations of dicamba on callus proliferation
To proliferate callus, nodular callus obtained from previous study was separated into two parts; non-chopping and chopping at 100 times (using 1 gFW callus) according to the method described by Heedchim et al. (Reference Heedchim, Te-chato and Yenchon2021). Then, 0.1 gFW of each sample was directly transferred to OPCM supplemented with different concentrations of dicamba (0, 0.1, 0.3, 0.5 mg/l) and 200 mg/l ascorbic acid. The cultures were placed at 26 ± 2°C under 10 h photoperiod (15 μmol/m2/s) provided by cool-white fluorescent lamps for 4 weeks. The diameter and characteristics of callus were investigated. A 2 × 4 factorial design in CRD with five replications was performed. Each replication consists of five test tubes and means among treatments were compared by DMRT at 1 or 5% probability. After 8 weeks of culture, SE was produced on the same medium.
Differentiation and morphological characteristics of SE and SSE
To induce SSE, SE obtained from 0.1 mg/l dicamba containing medium was transferred to MS medium without PGRs but modified by supplement with 0.2 M sorbitol together with 3% sucrose. After culture for 4 weeks, nodular callus proliferation and SSE at different stages were recorded under Leica stereomicroscope (EZ4W) and statistically compared.
Statistical analysis
The experimental data were analysed using R 2.14.0 software and subjected to one-way analysis of variance.
Results
Effects of different types and concentrations of auxins on callus induction
The IZE of mangrove fan palm cannot directly separate from endosperm after removing seed coat. So, it was firstly transferred to culture on medium without PGRs for 7–10 days to make IZE separate from endosperm. Then, IZE was transferred to medium with different types and concentrations of auxins. The results found that different types of auxins promoted callus induction at different frequency. Without and low concentration of 2,4-D at 1 mg/l could not induce callus formation. Increase in concentration of 2,4-D resulted in the increment of callus induction whereas increase in concentration of dicamba caused the decrement of callus induction. High concentration of 2,4-D at 5 mg/l or low concentration of dicamba at 1 mg/l gave 100% callus induction, significant difference with other treatments. For callus diameter, the results showed that 5 mg/l 2,4-D gave the highest result at 5.53 mm, not significant difference with other concentrations of 2,4-D but significant difference with all concentrations of dicamba. In case of callus characters, 5 mg/l 2,4-D provided yellow friable callus whereas 1 mg/l dicamba gave the yellow nodular callus (Table 1; Fig. 1(c)).
Table 1. Effects of different types and concentrations of auxins containing MS medium with 200 mg/l ascorbic acid on callus induction after culture for 8 weeks

** Significant difference (P < 0.01).
Mean values followed by the same letter within column are not significantly different according to DMRT.
Effect of embryo preparation on callus induction
Two different methods of IZE preparation resulted in different response in callus induction. Culturing of IZE without any preparation by cutting gave callus induction at 100% and callus diameter at 4 mm; whereas preparation of IZE by longitudinally cutting into half could not promote callus formation. The characteristics of callus were friable and yellow in colour. Moreover, cutting of IZE promoted browning of tissues leading to the failure of callus formation (Table 2).
Table 2. Effect of preparation of IZE and culture on solidified MS medium supplemented with 1 mg/l dicamba and 200 mg/l ascorbic acid for 8 weeks on callus induction

** Significantly different (P < 0.01) according to t-test.
Effects of chopping and concentrations of dicamba on callus proliferation
There are significant differences between non-chopped and chopped nodular callus on its proliferation. Chopping nodular callus gave the better result in callus diameter at 10.16 mm than non-chopped one which gave callus diameter at 9.48 mm. Among concentrations of dicamba tested, there were no significant differences but interaction between chopping and concentrations of dicamba was significant. The largest diameter of callus at 10.50 mm was obtained from chopping callus on 0.3 mg/l dicamba containing medium but there were no significant differences with 0.1 mg/l dicamba (low concentration) which gave the callus diameter at 10.33 mm. So, chopping nodular callus cultured on 0.1 mg/l dicamba containing medium was more suitable for callus proliferation of this plant (Table 3; Fig. 1(d)).
Table 3. Effects of chopping and concentrations of dicamba containing OPCM with 200 mg/l ascorbic acid on callus proliferation of mangrove fan palm after culture for 4 weeks

ns, No significant difference, *significantly different (P < 0.05).
Mean values followed by the same small and capital letter within row and column are not significantly different according to DMRT.
The morphology of nodular callus, primary and SSE is shown in Fig. 2. Nodular callus obtained on 0.1 mg/l dicamba containing medium originated from central and peripheral cells of the callus (Fig. 2(a)) after 4 weeks of culture and also produced SE in the same medium after 6–8 weeks. Upon transferring SE to MS medium with 0.2 M sorbitol, the SE developed into SSE that produced on primary SE within 4 weeks of culture (Fig. 2(b)). The SSE showed different stages of development; globular (Fig. 2(c) and (d)), fused SE (Fig. 2(b) and (c)) and haustorium embryo (Fig. 2(d)). The development of SE of mangrove fan palm was asynchronous. After 8 weeks of culture, some globular stages showed translucent and opaque colour with smooth edge and most of SSE presented in clusters; whereas some SSEs developed through haustorium stage that is whitish and light greenish colour. Moreover, the basal part of primary SE showed brown colour. Upon transferring SSEs to MS medium without PGR, torpedo-staged embryos (Fig. 2(e)) were developed and germinated into shoots at 40% without roots after 4 weeks of culture (Fig. 2(f)).
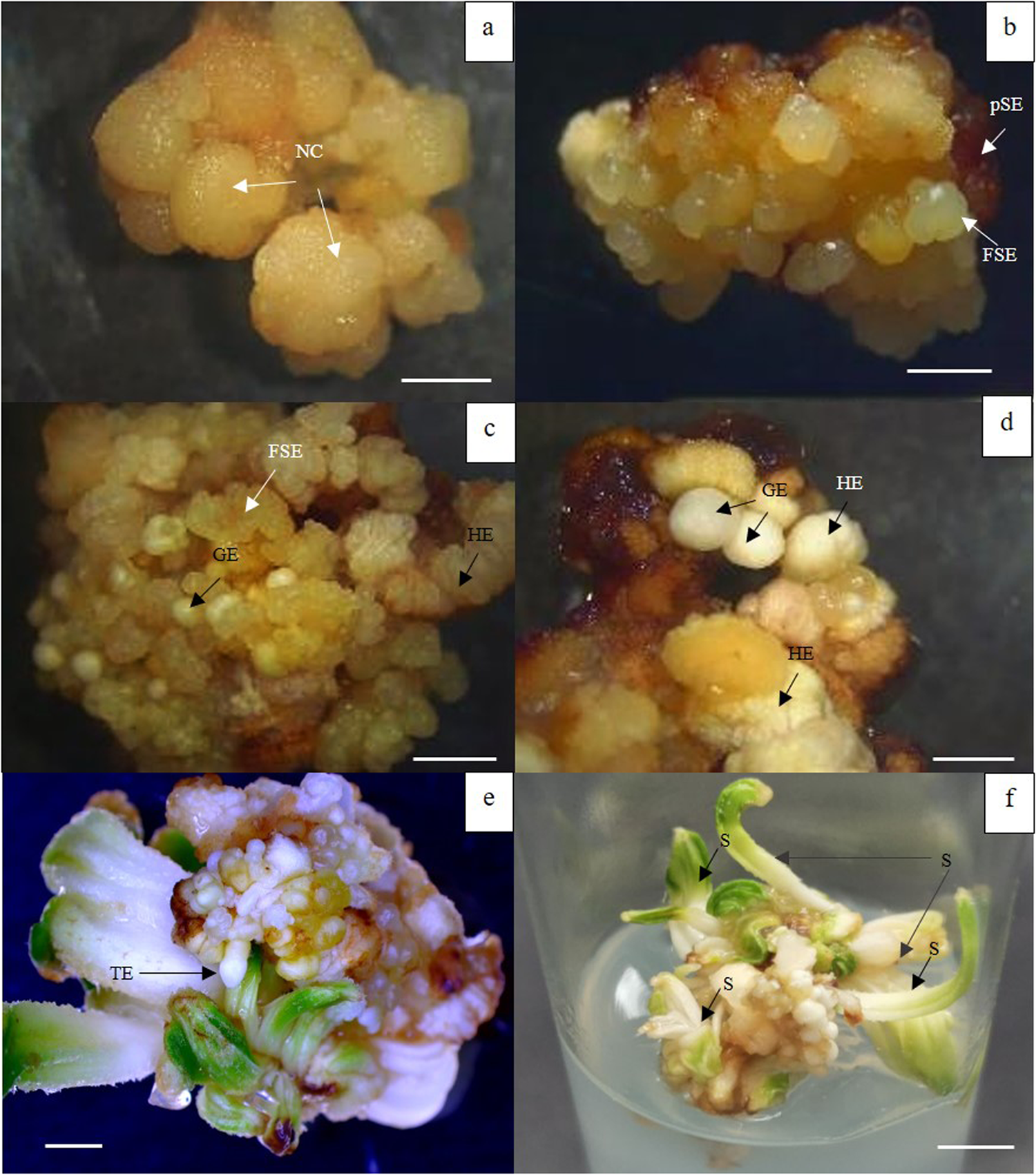
Figure 2. Development of somatic embryogenesis of mangrove fan palm under stereomicroscope (a–e) (bar = 2 mm). (a) Nodular callus (NC) obtained from OPCM with 0.1 mg/l dicamba for 4 weeks. (b–d) SSE at different stages produced from primary somatic embryo (pSE); globular embryo (GE), haustorium embryo (HE) and fused somatic embryo (FSE) occurred on 0.2 M sorbitol containing MS medium. (e, f) Germination of SSEs with torpedo embryo (TE) stage (bar = 2 mm) and shoot (s) on MS medium without PGR after 4 weeks of culture (bar = 5 mm).
Discussion
Auxins are a PGR that plays important role in somatic embryogenesis. Among types of auxins, 2,4-D and dicamba are considered the most effective and widely used to induce callus and SE in different palm tree species (Kanjanasopa et al., Reference Kanjanasopa, Somwong, Srisawat, Thitithanakul, Sontikul and Choengthong2017; Heedchim et al., Reference Heedchim, Te-chato and Yenchon2020; Niha et al., Reference Niha, Te-chato and Yenchon2022; Oliveira et al., Reference Oliveira, Mello, Araujo, Oliveira, Ferreira, Zanardo, Vieira, Otoni, Alexandre and Carvalho2022). In this work, 5 mg/l 2,4-D or 1 mg/l dicamba gave the highest callus formation frequency at 100% and callus diameter without significant difference. However, to avoid genetic variation, low concentrations of auxin are better for callus induction. When comparing the characteristics of callus, the results showed that 1 mg/l dicamba provided yellow nodular callus and underwent proliferation and differentiation into SE; whereas callus from other treatments showed browning in colour (data not shown). Culture medium without PGRs and low concentrations of 2,4-D at 1 mg/l could not induce callus but it promotes germination of shoot. Increasing concentrations of auxins caused oxidative reaction promoting the formation of phenolic compounds leading to tissue browning that is the main problem in tissue culture of palm trees. Similar result was reported by Ferreira et al. (Reference Ferreira, Silva-Cardoso, Meira and Scherwinski-Pereira2022a) and Oliveira et al. (Reference Oliveira, Mello, Araujo, Oliveira, Ferreira, Zanardo, Vieira, Otoni, Alexandre and Carvalho2022)
For preparation of IZE by longitudinal section before culturing, it provided negative effect on callus formation due to browning of explant as mentioned earlier. Therefore, IZE without cutting or wounding provided greater result in callus induction at 100% with diameter of 4 mm. Callus was characterized as nodular and yellow in colour. It was possible that cutting might destroy the meristematic tissues and inhibit cell division of IZE. In case of callus proliferation, the reduction of auxin concentration had a positive effect on callus proliferation from mature ZE of B. odorata as reported by Campos et al. (Reference Campos, Scherwinski-Pereira, Bernd, Fior and Schwarz2020) and also promoted SE formation in oil palm (Kerdsuwan & Te-chato, Reference Kerdsuwan and Te-chato2016; Heedchim et al., Reference Heedchim, Te-chato and Yenchon2020). In this study, significant differences in callus proliferation were not obtained among concentrations of dicamba tested but low concentration at 0.1 mg/l is optimum. Moreover, synergistic effect between chopping and concentration of dicamba (0.1 mg/l) had been found in the present study. Chopping is a physical stress in term of wounding that plays important role for cell division and differentiation of embryogenic cells (Othmani et al., Reference Othmani, Bayoudh, Drira, Marrakchi and Trifi2009) and it also increases surface contact of cell and culture medium leading to the increment of cell metabolism (Feher et al., Reference Feher, Pasternak and Duditis2003). In this study, 100 times of chopping followed by transferring to 0.1 mg/l dicamba containing culture medium was optimum for nodular callus proliferation like that reported by Heedchim (Reference Heedchim2019) in oil palm. Contrary results were obtained from Campos et al. (Reference Campos, Scherwinski-Pereira, Bernd, Fior and Schwarz2020) who reported that 450 μM picloram containing medium gave the highest primary callus formation at 84% and embryogenic callus formation at 36.33% in B. odorata.
For differentiation and development of SSE, in general, SE can germinate into new plant on medium without PGRs in many plant species. However, it is difficult in the palm tree. So, it was better to promote its germination through SSE formation as reported in oil palm (Heedchim et al., Reference Heedchim, Te-chato and Yenchon2021; Niha et al., Reference Niha, Te-chato and Yenchon2022). In brief, SE were cultured on the medium with sorbitol for a certain period of time to induce SSE followed by transferring those SSE to PGR-free medium. Secondary somatic embryogenesis is a process whereby new SE, the so-called SSE, is initiated from originally formed primary SE and has certain advantages compared to primary somatic embryogenesis such as very high multiplication rate, independence of an explant source and repeatability. Additionally, embryogenic capacity can be maintained for a long period of time by repeated cycles of secondary embryogenesis. SSE has the potential to produce many plants and produce embryos over a long period of time (Te-chato and Hilae, Reference Te-chato and Hilae2007). Sorbitol is sugar alcohol that decreases the water content in the cells and promotes the maturation of embryos and also SSE in many plant species including palm tree (Te-chato and Hilae, Reference Te-chato and Hilae2007; Sanputawong and Te-chato, Reference Sanputawong and Te-chato2011; Heedchim et al., Reference Heedchim, Te-chato and Yenchon2021). Oil palm SSE formation was achieved on 0.2 M sorbitol containing MS medium (Sanputawong and Te-chato, Reference Sanputawong and Te-chato2011). Moreover, Te-chato and Hilae (Reference Te-chato and Hilae2007) found that among carbon source tested, 0.2 M sorbitol gave the best results in SSE formation with 100% and 21.55 embryos/SE. Asynchronous SSE was observed under the stereomicroscope in this present study. Different stages of SSE including translucent and opaque globular and fused SE were obtained and clearly detected like those obtained in E. precatoria (Ferreira et al., Reference Ferreira, Silva-Cardoso, Meira, Costa and Scherwinski-Pereira2022b). However, the tissue culture of palm tree is still a challenging process that is affected by many biological and physiological factors. So far, there is no report about tissue culture of mangrove fan palm. This is the first report on in vitro propagation of mangrove fan palm that is basically successful in induction of callus, SE, SSE and SSE germination. However, the germination of SSE was low (about 40% with shoot only) due to asynchronous development or imbalance of PGRs added in culture medium. There was no successful root induction, so this research is still in progress. Thus, important factors affecting those processes, especially PGR in term of auxin, strength of medium and activated charcoal need to be further investigated. By the success of this protocol, it is very important for mass propagation and genetic conservation of mangrove fan palm in the future.
Conclusions
Whole part of IZE without wounding was suitable initial explant for in vitro propagation of this plant. The best callus induction was obtained on 1 mg/l dicamba containing MS medium. Well-proliferated callus and SE formation occurred on OPCM with reduced concentration of dicamba to 0.1 mg/l. SSE at various stages of development was found on 0.2 M sorbitol containing MS medium. The germination of those SSEs at 40% was obtained on medium without PGRs. Further root induction is in progress.
Acknowledgements
This research is supported by Royal Project under the title of micropropagation of fan palm (Licuala spinosa Thunb.) and its conservation in vitro. We would like to thank the Center of Excellence in Agricultural and Natural Resources Biotechnology (CoE-ANRB): Phase 3, Agricultural Innovation and Management Division, Faculty of Natural Resources and Graduate School, Prince of Songkla University, Thailand for supporting facilities used in this research. Special thanks to Dr Narit Taochan for giving us permission to use the stereomicroscope for recording pictures and Dr Leslie Stephen Jenning for proof reading and valuable suggestion.
Author contributions
S. T. designed the experiments, proof-read and validated all the content of the manuscript. W. H. performed and analysed the experiments. C. N. and S. Y. reviewed drafts of the manuscript. All authors read and approved the manuscript for publication.
Competing interest
None.









