No CrossRef data available.
Article contents
Resistance of sugarcane hybrids to internode borer Chilo sacchariphagus indicus (Lepidoptera: Crambidae)
Published online by Cambridge University Press: 21 February 2024
Abstract
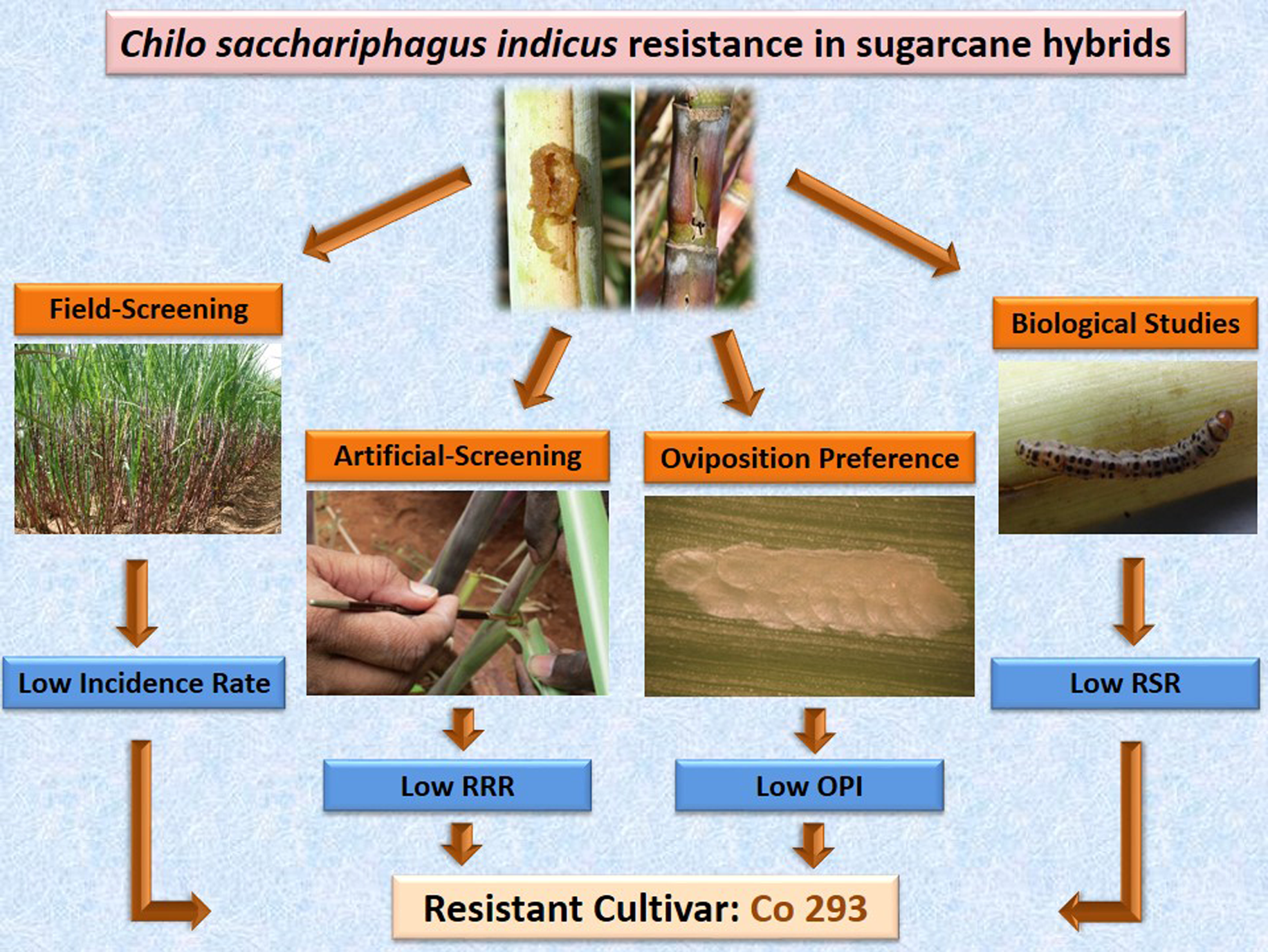
A four-year field study (2013–2016) was conducted to screen Indian sugarcane hybrids together with two susceptible checks against internode borer Chilo sacchariphagus indicus (Kapur) (Lepidoptera: Crambidae) in endemic locations of Tamil Nadu State, India. Each year, borer incidence on cane basis and intensity on internode basis were assessed at harvest to eliminate susceptible entries. Of the total 535 hybrids screened, only Co 293 emerged as resistant at the end of fourth year trial which was confirmed in tests under controlled conditions with artificial infestation. A modified relative resistance ratio computed using incidence and intensity also confirmed its resistance to the borer. In laboratory oviposition choice tests with excised leaves of the resistant Co 293 and susceptible hybrids Co 86032 and Co 1060, percent of leaf bits oviposited, egg masses laid, and egg numbers deposited were significantly lowest in Co 293. Also, an oviposition preference index computed for both egg mass number and egg number was significantly lowest for Co 293 which suggested antixenosis. Larval survival was significantly lowest in Co 293 with 5 to 10-fold higher neonate mortality than in the two susceptible hybrids. Prolonged larval development period and lower fecundity were observed when the borer was reared on Co 293 which indicated antibiosis. A relative suitability ratio developed from larval and pupal durations also indicated lower suitability of Co 293. Among the plant morphological characters examined, leaf length and cane thickness positively influenced borer incidence; loose sheath-clasp was associated with higher borer incidence. Among 12 shoot phenolics quantified, eight were present in higher quantities in Co 293 suggesting their role in antibiosis. Co 293 identified as resistant hybrid in the present study has the potential to be used as a parent in breeding programs for C. sacchariphagus indicus resistance.
Keywords
- Type
- Research Article
- Information
- Copyright
- © The Author(s), 2024. Published by Cambridge University Press





